编者按:近年来,随着多款靶向、免疫药物及其联合方案的不断涌现并取得突破性进展,肝细胞癌的系统治疗迎来了巨大变革,从靶向或免疫单药进入免靶或双免联合的免疫联合时代。
在2025年1月23日召开的的美国临床肿瘤学会胃肠道肿瘤研讨会(ASCO GI 2025)上,备受关注的CheckMate 9DW研究和CARES-310研究均重点关注了未接受治疗的不可切除肝细胞癌(uHCC)患者双免治疗的效果以及病毒感染状态对双免治疗的影响(摘要号:520和摘要号:578)。《肿瘤瞭望消化时讯》特将该两项研究进行了整理,并荣幸地邀请了中国人民解放军东部战区总医院刘秀峰教授针对上述研究进行了深度剖析与点评。现将内容整理如下,以飨读者。

刘秀峰 教授
东部战区总医院秦淮医疗区肿瘤科副主任
主任医师、医学博士
国家卫健委能力建设和继续教育肿瘤学专委会 秘书
国家癌症中心肝癌质控专家委员会 委员
CSCO 肝癌专家委员会 副主任委员兼秘书长
CSCO 胆道肿瘤专家委员会 副主任委员
CSCO 胃肠间质瘤专家委员会 常务委员
中国抗癌协会肝癌专业委员会 常务委员
中国肝癌精准治疗联盟专家委员会 常务委员
第四届国之名医·优秀风范
研究简介
01CheckMate 9DW研究在Ⅲ期CheckMate 9DW研究(NCT04039607)中,与仑伐替尼(LEN)/索拉非尼(SOR)相比,一线纳武利尤单抗(NIVO)+伊匹木单抗(IPI)在未接受治疗的uHCC患者中显示出显著的总生存期(OS)获益和更高的客观缓解率(ORR),并且缓解持久,安全性可控。本次大会报告了按最佳总缓解(BOR)亚组和基线特征划分的疗效,以及预先计划的期中分析中的额外安全性分析。
研究者将不符合根治性手术或局部治疗条件的、Child-Pugh评分为5或6、ECOG评分为0或1的既往未接受系统治疗的肝细胞癌患者按1:1随机分配,接受每3周(Q3W)一次纳武利尤单抗1 mg/kg + 伊匹木单抗3 mg/kg(最多4个周期),然后纳武利尤单抗480 mg每4周(Q4W)一次,或仑伐替尼8 mg或12 mg每日一次(QD),或索拉非尼400 mg每日两次(BID),直至疾病进展或出现不可耐受的毒性。纳武利尤单抗最多给药2年。主要终点是OS;次要终点包括由盲态独立中心(BICR)根据实体瘤疗效评价标准1.1版(RECIST v1.1)评估的ORR和缓解持续时间(DOR)。
结果显示,共有668例患者被随机分配至纳武利尤单抗+伊匹木单抗组(n=335)或仑伐替尼/索拉非尼组(n=333)。在中位随访时间35.2个月时,纳武利尤单抗+伊匹木单抗组的中位OS为23.7个月,仑伐替尼/索拉非尼组为20.6个月(HR 0.79,95%CI:0.65~0.96,P=0.0180)。此外,纳武利尤单抗+伊匹木单抗组ORR也显著高于仑伐替尼/索拉非尼组(36% vs. 13%;P<0.0001),中位DOR分别为30.4个月和12.9个月。
在24周这一关键时间点,纳武利尤单抗+伊匹木单抗组相较于仑伐替尼/索拉非尼组在所有BOR亚组中均观察到生存获益(表1)。在亚组分析中,无论肝细胞癌病因(未感染:35% vs. 8%;HBV感染:25% vs. 17%;HCV感染:50% vs. 16%)如何,或无论是BCLC分期为≤B期(33% vs. 13%)或C期(37% vs. 14%)的患者,纳武利尤单抗+伊匹木单抗组ORR均高于仑伐替尼/索拉非尼组。安全性数据见表1。未来将展示额外的探索性分析。
表1.两组生存数据与安全性数据
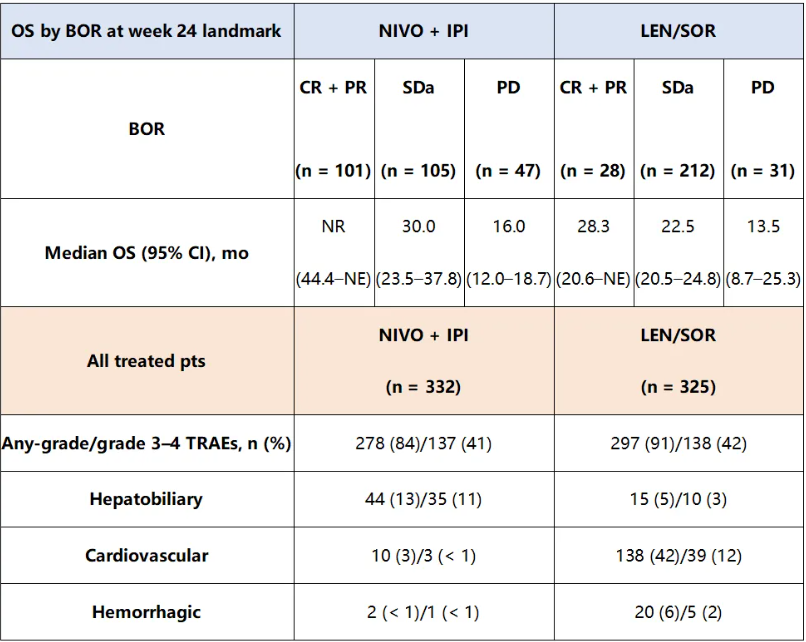
总之,CheckMate 9DW的这些额外分析证明了在未接受系统治疗的肝细胞癌患者中一线纳武利尤单抗+伊匹木单抗的疗效和可控的安全性,进一步巩固了该方案在肝细胞癌一线治疗中的地位。
02CARES-310研究
CARES-310研究(NCT03764293)评估了PD-1抑制剂卡瑞利珠单抗与VEGFR 1-3抑制剂阿帕替尼联用相比索拉非尼治疗初治uHCC的疗效。与索拉非尼相比,卡瑞利珠单抗+阿帕替尼显著提高了中位OS和中位PFS(中位OS:23.8个月 vs. 15.2个月;中位PFS:5.6个月 vs. 3.7个月;P<0.0001)。卡瑞利珠单抗+阿帕替尼组中,最常见(≥10%)的≥3级治疗相关不良事件为高血压(38.6%)和天冬氨酸氨基转移酶(AST)升高(20.2%)。本次大会公布的数据是对CARES-310研究进行事后分析,使用Kaplan-Meier方法估计中位OS和中位PFS,并使用log-rank检验比较非病毒性、丙型肝炎病毒(HCV)和乙型肝炎病毒(HBV)三种病因组之间的差异。
研究结果显示,无论是在非病毒性(HR:0.68,95% CI:0.39~1.19)、HCV(HR:0.37,95%CI:0.162~0.84)还是HBV(HR:0.70,95%CI:0.55~0.89)病因的患者中,卡瑞利珠单抗+阿帕替尼组的中位OS和中位PFS均长于索拉非尼组(表2)。
表2. 不同病毒亚组生存数据
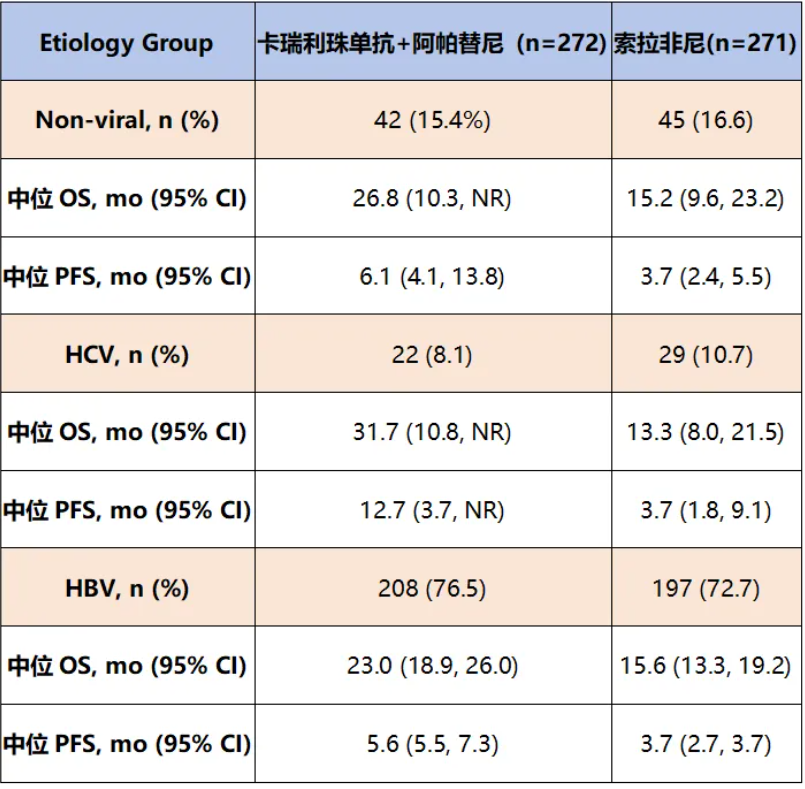
总之,CARES-310研究显示,无论是非病毒性还是病毒性肝细胞癌患者,卡瑞利珠单抗+阿帕替尼相比索拉非尼具有临床上有意义的mOS获益,为无论病因如何的肝细胞癌患者一线治疗提供了临床获益的证据。
专家点评
一直以来不敢轻易点评某项研究,如同定义历史人物,不了解时代背景、不知晓人物的心路历程和社会关系,解读或许只能庸人自扰,贻笑大方。临近乙巳年春节,借此平台先祝学界各位同道新年快乐,诸事顺遂!
CheckMate 9DW和CARES-310都已进入指南,其中CheckMate 9DW对标HIMALAYA,CARES-310对标LEAP002。很长一段时间,uHCC患者一线治疗的优化选择都是学界的困惑、“幸福的烦恼”,笔者试着做个向导,带领大家走进2025 ASCO GI 的摘要520和578,领略“双免”和“靶免”的风采。
可能没有“好事者”会比较CheckMate 9DW与CARES-310的对照组。CheckMate 9DW的对照组85%以上应用的是仑伐替尼(n=333),而CARES-310对照组则全部是索拉非尼(n=271),对照组的mOS分别是20.6个月 vs. 15.2个月,PFS分别为9.2个月 vs. 3.7个月,ORR分别为13% vs. 5.9%。似乎CheckMate 9DW和CARES-310也在重演7年前REFLECT的剧本[1],但是2017年HCC领域免疫治疗没有获批,REFLECT的非劣效(HR=0.92)着实很辛苦,仑伐替尼 vs. 索拉非尼的数据分别是OS 13.6个月 vs. 12.3个月、PFS 7.4个月 vs. 3.7个月,ORR 24% vs. 9.2%。单从TKI的角度审视uHCC的系统治疗,只能说今非昔比了,TKI赶上了好时代,有免疫治疗的加持,无论近期还是远期疗效都有了显著提高。
换个视角,试问一下外科临床医生会偏好双免抑或靶免吗?“O+Y”的ORR为36%(包括7%的CR),“双艾”的ORR为25.4%,mOS都逼近2年。就uHCC的一线治疗而言,2年的时间截点很快将被突破,MDT团队成员都在跃跃欲试,因为可以用“时间换空间”。CheckMate 9DW可能回答了一个问题,BOR的患者(即便是SD,也有30个月的生存,而CR/PR者生存期尚未达到)在“挡刀”?!这个场景HIMALAYA也同样呈现过,是否暗示双免在辅助治疗中的价值(原谅笔者走火入魔、天马行空)?虽然靶免较双免ORR略低,但快速的起效和迅速分离的OS曲线似乎在召唤外科临床医生,相应的转化研究在中国大陆如雨后春笋。当然,药效经济学和可及性也是重要的考量。
CheckMate 9DW和CARES-310本次还不约而同回答了一个有趣的问题,免疫治疗2.0版可以覆盖HCC的病因(9DW HBV/HCV/NBNC分别为:34%/27%/37%,CARES-310分别为:76.5%/8.1%/15.4%)。虽然不同病因的亚组OS不同,但都较对照组明显延长。早先的荟萃分析发现,NBNC的HCC患者不能从免疫治疗中获益[2]。这个观点是全球著名的Josep M. Llovet教授提出的,笔者一直跟随、深信不疑。那CheckMate 9DW和CARES-310有关病因的论断错了吗?Josep M. Llovet的观点主要来源于对CheckMate 459和KEYNOTE 240数据分析,而免疫单药的时代已经或即将成为历史,CTLA-4、VEGFR、TIGIT以及局部治疗(如TACE、HAIC、SBRT)等角色的“友情助演”,可以激活肝癌免疫微循环的多环节、多步骤。甚至有比较极端的观点,“免疫耐药”是个伪命题(非笔者一家之言)!
滑动查看摘要原文:
摘要520
Background:In the phase 3 CheckMate 9DW study (NCT04039607), 1L NIVO + IPI demonstrated significant overall survival (OS) benefit vs LEN/SOR, higher objective response rate (ORR) with durable responses, and manageable safety in uHCC. We present efficacy by best overall response (BOR) subgroups and baseline characteristics, and additional safety analyses from the preplanned interim analysis.
Methods:Patients (pts) with previously untreated HCC not eligible for curative surgical or locoregional therapies, Child-Pugh score 5 or 6, and ECOG performance status 0 or 1 were randomized 1:1 to receive NIVO 1 mg/kg + IPI 3 mg/kg Q3W (up to 4 cycles), then NIVO 480 mg Q4W or LEN 8 mg or 12 mg QD or SOR 400 mg BID until disease progression or unacceptable toxicity. NIVO was given for a maximum of 2 years. The primary endpoint was OS; secondary endpoints included ORR and duration of response (DOR) per blinded independent central review (BICR) using RECIST v1.1.
Results:A total of 668 pts were randomized to NIVO + IPI (n = 335) or LEN/SOR (n = 333). At a median follow-up of 35.2 (range 26.8–48.9) months (mo), median OS (95% CI) was 23.7 (18.8–29.4) mo with NIVO + IPI vs 20.6 (17.5–22.5) mo with LEN/SOR (HR 0.79 [95% CI 0.65–0.96];?P?= 0.0180). ORR (95% CI) per BICR was significantly higher with NIVO + IPI vs LEN/SOR (36% [31–42] vs 13% [10–17];?P?< 0.0001); median DOR (95% CI) was 30.4 (21.2–not estimable [NE]) mo vs 12.9 (10.2–31.2) mo. Survival benefit of NIVO + IPI vs LEN/SOR was observed across BOR subgroups at the 24-week landmark timepoint (Table). In subgroup analyses, ORR (95% CI) per BICR was higher with NIVO + IPI vs LEN/SOR across HCC etiologies (uninfected: 35% [26–44] vs 8% [4–15]; HBV infected: 25% [17–34] vs 17% [10–25]; HCV infected: 50% [39–61] vs 16% [9–25]) and in pts with Barcelona Clinic Liver Cancer stage ≤B (33% [23–43] vs 13% [6–21]) or stage C (37% [31–44] vs 14% [10–19]). Safety data are shown in the Table. Additional exploratory analyses will be presented.
Conclusions:These additional analyses from CheckMate 9DW demonstrate the efficacy and manageable safety of 1L NIVO + IPI in uHCC and further support its use as a potential standard-of-care treatment option in this setting.
aIncludes non-CR/non-PD. CR, complete response; NR, not reached; PD, progressive disease; PR, partial response; SD, stable disease; TRAE, treatment-related adverse event.
摘要:578
Background:CARES-310 (NCT03764293) evaluated the combination of PD-1 inhibitor, camrelizumab (cam), and VEGFR-1-3 inhibitor, rivoceranib (rivo), compared to sorafenib (sor) for the treatment of uHCC. Cam + rivo significantly improved median overall survival (mOS) and median progression-free survival (mPFS) compared to sor (mOS, 23.8 months [mo] [95% CI 20.6, 27.2] vs 15.2 mo [95% CI 13.2, 18.5] hazard ratio [HR] 0.64 [95% CI 0.52, 0.79]; one-sided p<0.0001; mPFS, 5.6 mo [95% CI 5.5, 7.4] vs 3.7 mo [95% CI 3.1, 3.7]; HR 0.54 [95% CI 0.44, 0.67]; one-sided p<0.0001). The most common (≥10%) grade ≥3 treatment-related adverse events in the cam + rivo arm were hypertension (38.6%) and AST increased (20.2%).
Methods:A post-hoc analysis of CARES-310 was performed, where mOS and mPFS were estimated using the Kaplan-Meier method and compared between the 3 etiology groups of non-viral, hepatitis C virus (HCV), and hepatitis B virus (HBV) using the log-rank test.
Results:mOS was longer with camrelizumab plus rivoceranib compared with sorafenib in patients with non-viral (HR 0.68 [95% CI 0.39, 1.19]), HCV (HR 0.37 [95% CI 0.162, 0.84]), and HBV etiologies (HR 0.70 [95% CI 0.55, 0.89]) (Table). Similarly, mPFS was longer with camrelizumab plus rivoceranib compared with sorafenib in patients with non-viral (HR 0.55 [95% CI 0.34, 0.91]), HCV (HR 0.50 [95% CI 0.23, 1.06]), and HBV etiologies (HR 0.57 [95% CI 0.45, 0.72]) (Table).
Conclusions:Cam + rivo in CARES-310 suggested clinically meaningful mOS benefit in non-viral and viral HCC vs sor and provides assurance of clinical benefit for first line treatment to patients with uHCC independent of etiology.
HCC, hepatocellular carcinoma; mOS, median overall survival; mPFS, median progression-free survival; mo, months; HR, hazard ratio; HCV, hepatitis C virus; HBV, hepatitis B virus, NR, not reached.
Results used Cox proportional hazard model.
Confidence Intervals (CI) used the Brookmeyer and Crowley method.
参考文献
1.Lancet. 2018,391(10126): 1163-1173.
2.Nature Reviews Gastroenterology & Hepatology,2023,20:487–503.



